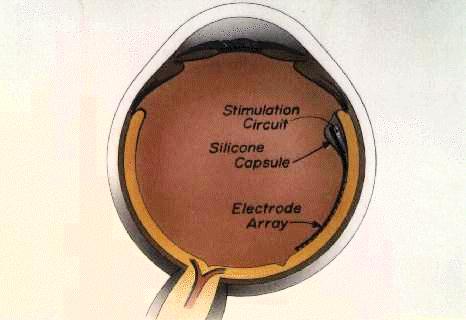
Massachusetts Eye and Ear Infirmary, Draper Laboratories, MIT-Lincoln Laboratories, Massachusetts Institute of Technology
Purpose. Development of an epiretinal prosthesis with mechanical properties that would promote firm adhesion to the retina without significant downward pressure against the ganglion cells.
Methods. Epiretinal implantation following standard vitrectomy incited contraction of cortical vitreous in 24 of 29 rabbits. Therefore, a surgical method was sought to achieve wide-field removal of cortical vitreous, which was assessed with scanning and transmission electron microscopy. To obtain the proper contour, a cantilever was molded to match the retinal curvature (determined by MRI). A strain gauge measured downward forces generated by the cantilever. For the purpose of adhesion, polymers of varying hydration were tested in vitro along the inner retinal surface.
Results. A manual method was developed for stripping cortical vitreous, which was verified by electron microscopy in two animals. Downward pressure (distributed over 0.5 sqmm) of less than 1.1 mmHg was measured at the end of the cantilever. Firm adhesion of the implant to the retina was dependent upon removal of cortical vitreous and choice of polymer -- more hydrophilic polymers were preferable.
Conclusions. The retina can be denuded of cortical vitreous over a wide area in the rabbit, which ensures close apposition of the cantilever (which houses the stimulating electrodes) to the ganglion cells and eliminates the potentially adverse effects of retained cortical vitreous. The cantilever with attached polymer can provide firm adhesion with very low downward pressure. These properties are essential for the proper functioning of a chronic epiretinal prosthesis.

Figure 1
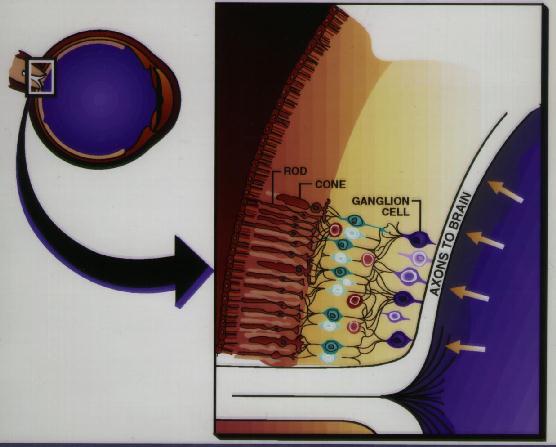
Figure 2
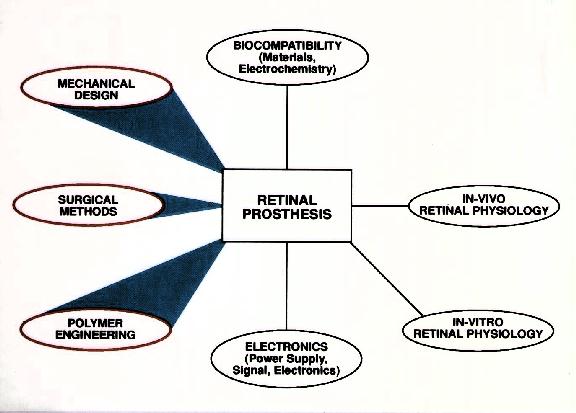
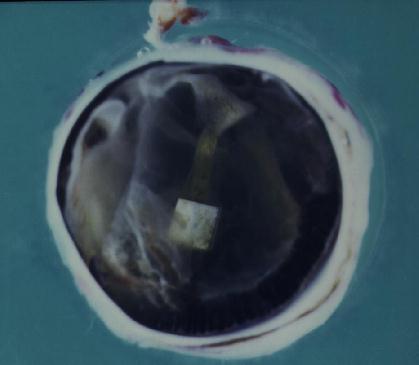
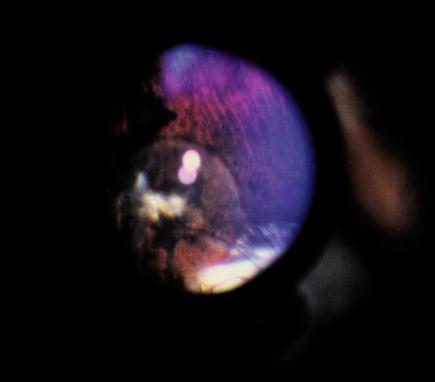
Figure 3 Surgeries to implant an epiretinal prosthesis were initially performed with "dummy" (non-electrically active) implants in Dutch-belted rabbits to learn methods that would not damage the retina. In 24 of 29 technically successful cases, epiretinal implantation incited profound intracular inflammation in the worst cases (Figure 4) or contraction of the cortical vitreous, which lifted the implant frmo the retina and caused a retinal detachment. Inexplicably, the post-operative course of five cases was stable (Figure 5), up to 17 months in the best case. The most common outcome was a post-operative course with relatively little imflammation followed days later by contraction of cortical vitreous and retinal detachment. Therefore a surgical method was sought to dissect the cortical vitreous from a wide area of the posterior retina.
The degree to which cortical vitreous was removed without damaging the retina was assessed by scanning and transmission electron microscopy. Two surgeries were performed in which a "core" vitrectomy was performed with a rotary vitrector, which permits relatively close shaving along the retinal surface. Stripping of the cortical vitreous was then performed over one-half of the posterior pole. The eyes were placed in fixative immediately following surgery and studied histologically.
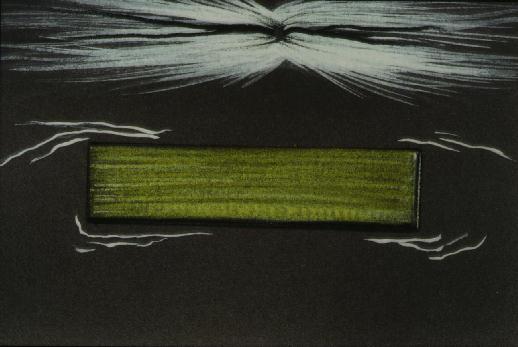
Figure 6 Placement of polymide on retinal surface intiates contraction of cortical vitreous.
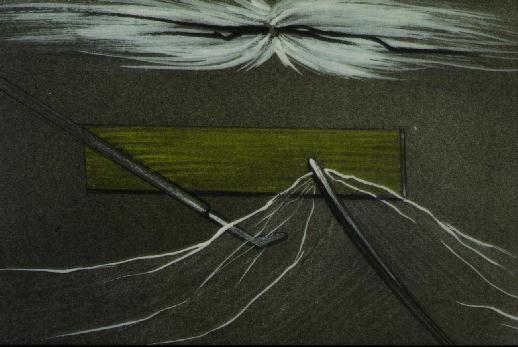
Figure 7 The now-visible strands of cortical vitreous are grasped and reflected. Blunt dissection in pre-retinal space can be safely performed.

Figure 8 The retinal surface is denuded of cortical vitreous. The "dummy" implant is removed.

Figure 9 Scanning electron micrograph (magnification 1200x) of the boundary between regions where stripping of cortical vitreous had and had not been performed (right and left sides, respectively) in the rabbit. The retinal surface is smooth in regions denuded of cortical vitreous.
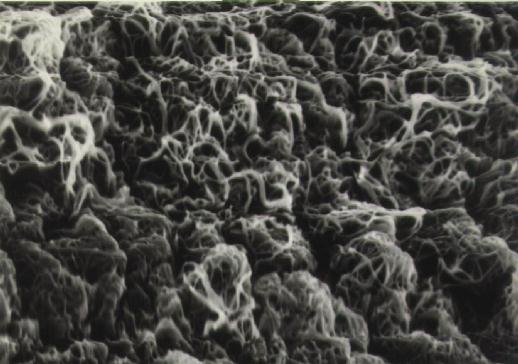
Figure 10 Magnified view (4500x) of left side of above micrograph. The retina is covered with strands of cortical vitreous.
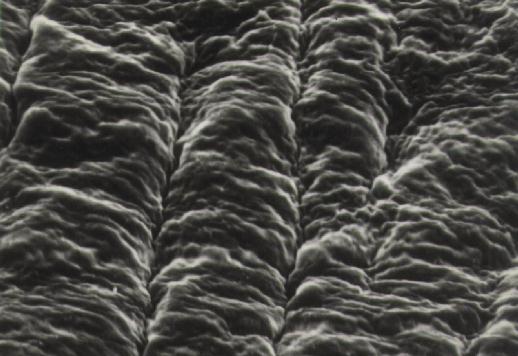
Figure 11 Magnifed view (4500x) of right side of above micrograph. The retina appears smooth because cortical vitreous is not present.
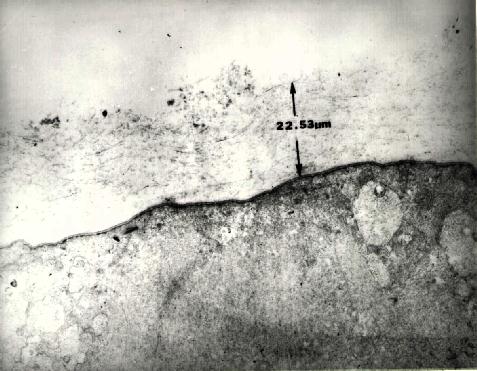
Figure 12 Transmission electron micrograph (15,600x) of retina in a region where only a core vitrectomy had been performed. A 22.5 micron layer of cortical vitreous remains.
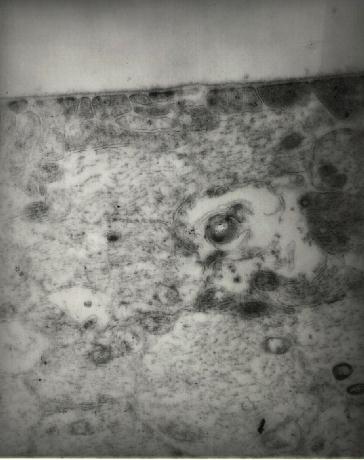
Figure 13 Transmission electron micrograph (46,500x) of retina in a region where stripping of cortical vitreous had been performed. The inner limiting membrance is devoid of cortical vitreous and does not appear to be damaged.
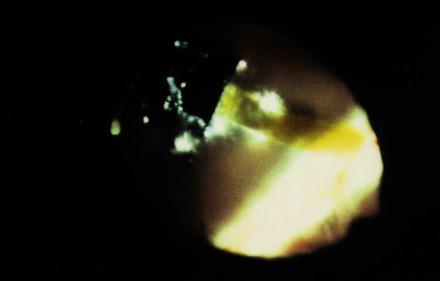
Figure 14 Intraoperative photograph of first successful epiretinal placement of a "dummy" implant following stripping of cortical vitreous.
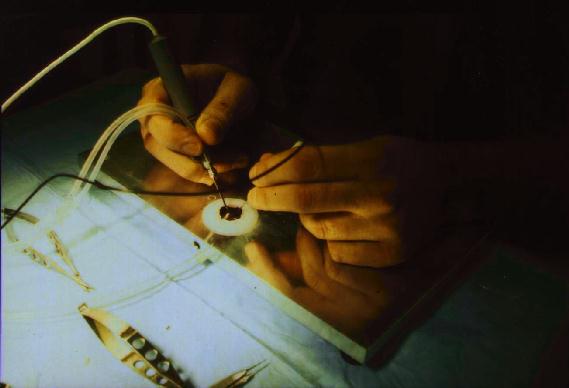
Figure 15 An artificial orbit is used to hold the eye and suction is applied to maintain the external coutour after removal of the anterior segment. A core vitretomy is performed and implants with varying radii of curvature can be placed on the retina to assess the adequacy of the fit. Iterative trial-and-error is used to improve the design.
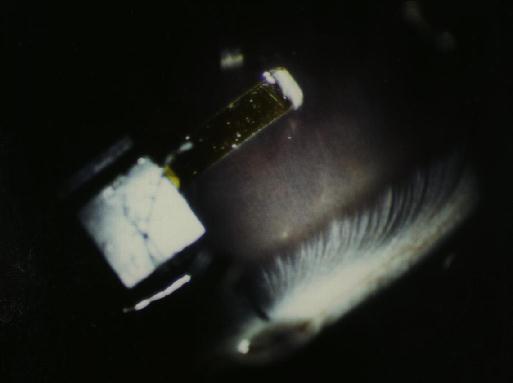
Figure 16 An example of the benefit of the artificial orbit. An implant which was thought to be properly contoured gouges the retina (arrow). This error can be detected with the artificial orbit without having to perform surgery. This strategy has the advantage of reducing the number of animals that are needed to improve this aspect of the design.
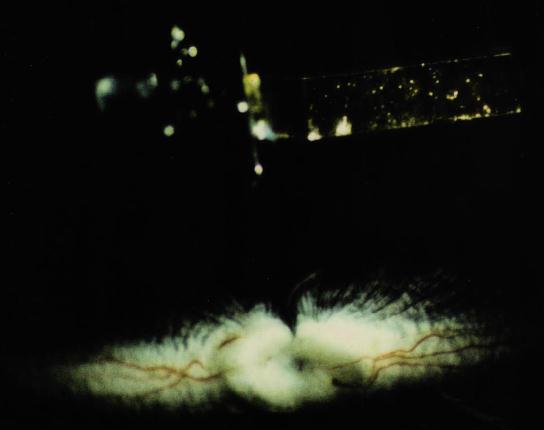
Figure 17 This implant has a radius of curvature that more closely approximates the retinal surface.
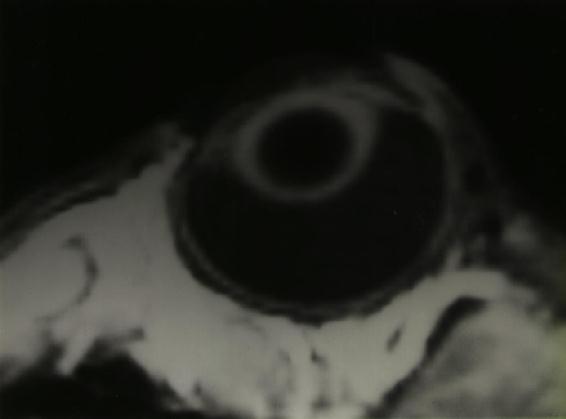
Figure 18 Magnetic resonance image of a rabbit eye. The digitized data can be used to determine the inner radius of curvature of the retina at the desired site of implantation.

Figure 19 Magnetic resonance data is used to construct a mold matching the estimated post-operative curvature of the retina. Implants with varying curvatures are made by molding the cantilever with silicone. The silicone chips are encapsulated in silicone (arrow), which provides a seal to protect the chips from salt, which is lethal to silicon.

Figue 20 The polyimide cantilever (yellow) is 50 microns thick and therefore razor-sharp on edge. Silicone is used to coat the edges to protect the retina during insertion. Coating the edges helps limit damage like that pictured above where the cantilever was not coated with polymer.
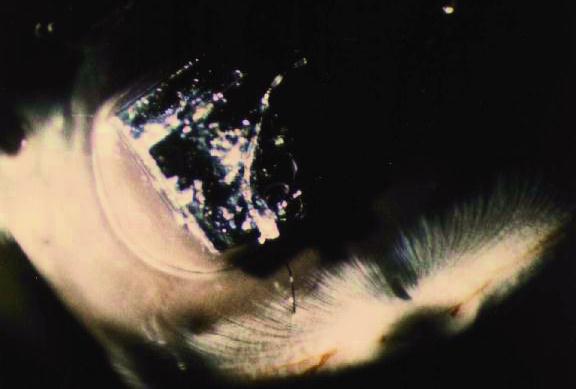
Figure 21 Hydrogel (arrow) positioned under the square silicon chips provides the necessary adhesion to the retina without glues, tacks or lasers.
Figure 22 The effectiveness of the hydrogel bond can be assessed in the artificial orbit. A suture is adhered to the implant and the force ("Y" axis) generated by vertical displacement ("X" axis) can be quantified. This graph shows results from one experiment. Two trials produced similar curves.
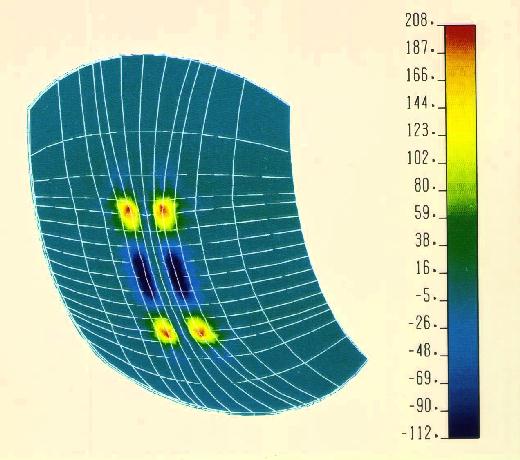
Figure 23 3-Dimensional finite element analysis of downward force on the retina generated by a polyimide cantilever, using the following assumptions:
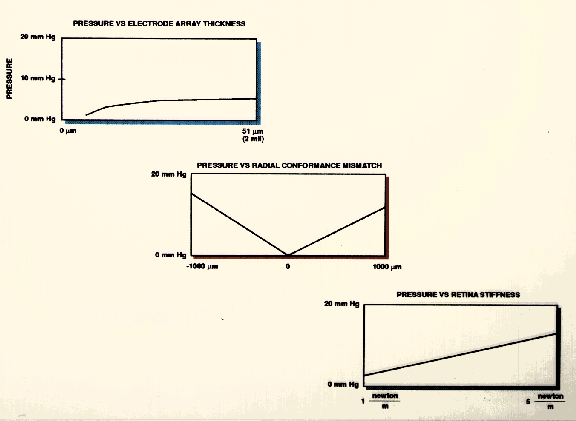
Figure 24 Parametric analysis of downward force ("Y" axis) produced by:
These factors make it necessary to remove cortical vitreous prior to epiretinal placement of an implant, at least for an implant with these mechanical properties. A surgical method for removal of cortical vitreous was sought because of the absolute need to know if the retinal surface is denuded of cortical vitreous prior to implantation.
The vitreous of the rabbit is more reactive than that of humans and most other species. It might not be necessary to strip cortical vitreous in species other than rabbit.
The methods described in this poster provide an approach to meet each of these goals.
The question of biocompatibility is the real essence of the project, and we have studied this issue in more depth in a separate study. Each of the materials used in our implant are individually placed into the vitreous cavity. Each animal is prospectively observed for at least one year with clinical examinations and electroretinograms. Eventually histology of the retina is performed.
A separate line of inquiry is also underway to assess the adequacy of the seal provided by silicone encapsulation around the silicon chips. This study consists of measuring changes in impedance when the implant is chronically submerged in balanced salt solution.