MASSACHUSETTS INSTITUTE OF TECHNOLOGY
ARTIFICIAL INTELLIGENCE LABORATORY
A.I.Memo No. 1330
November, 1991
Review of Artificial Muscle based on Contractile Polymers
David L. Brock
Abstract
An artificial muscle with strength and speed equal to that of a human
muscle may soon be possible. Polymer gels exhibit abrupt volume changes
in response to variations in their external conditions --- shrinking or
swelling up to 1000 times their original volume. Through the conversion
of chemical or electrical energy into mechanical work,
a number of devices have already been
constructed which produce forces up to 100N/cm2 and contraction
rates on the order of a second. Though the promise of an artificial
muscle is real, many fundamental physical and engineering
questions remain before the extent or limit of these devices is
known.
1 Introduction
The electric motor is the primary actuator used in robotics. However, its
high weight, limited sizes, complex transmissions and restrictive shapes
have constrained the design and development of robotic systems. Alternatives
such as pneumatics and hydraulics have achieve some successes, but these
depend on compressors which are themselves driven by electric motors.
Nitinol, a shaped memory alloy, has also been used in robotics, however
the thermal to mechanical energy conversion has proven difficult in
practical applications.
What would be ideal for robotic actuation would be an analog of a
human muscle --- a contractile compliant material driven by chemical
or electrical signals. Recent research in polymer gels offers the
hope for an artificial muscle and a potential
revolution in robot actuator design.
Gel is intermediate between liquid and solid, consisting of a polymer
network and interstitial fluid. The properties of the gel, including
its equilibrium and dynamic aspects, are defined by the interaction
between the polymer and the liquid. Examples of gels abound including
natural gels, such as Jello, the vitreous humor of the eye, the lining
of the stomach, intestine and lung, and muscle; and artificial gels,
such as polyacrylamide, polystyrene, and others used in the
manufacture of rubber, plastics, glues and films. A property common to
all gels (Li 1989) and one important for actuator design is their
unique ability to undergo abrupt changes in volume.
Gels can swell or shirk as much as 1000 times in response to small
changes in external conditions such as temperature, pH, electric
fields (De Rossi 1986, Tanaka 1982), or solvent and ionic composition
(Tanaka 1987). Not only are these changes large, they are also
reversible. Restoration of the initial external conditions returns
the gel to its original volume. In general, the rate of contraction is
proportional to the square of the linear dimension of the gel. For
example, micro sized gel fibers contract in milliseconds. In
addition, some gels support substantial
loads. Polyacrylonitrile-polypyrrole (PAN-PPY) and polyvinylachohol
(PVA) gel fibers generate up to 100N/cm2 (Chiarelli 1989),
approximately equal to that of a human muscle.
The possibility of an artificial muscle is quite exciting. In fact a
number of researchers have already constructed
robotic and prosthetic prototypes based on contractile gels. However,
to move beyond a mere laboratory curiosity, a number of fundamental
issues must be addressed, such as efficiency, power density, energy storage and
transmission, dynamics, control, heat dissipation, actuator design,
and others. In addition, contractile gel actuators must compared
favorable to existing actuators, at least in some applications, to be
considered a viable alternative in mechanical design.
This review outlines some of the basic research in polymer gels,
providing a background for the analysis and design of gel actuators.
First, the physical mechanisms of gel volume change are discussed. Second,
the processes and methods of gel preparation are presented. Third, the
gel kinetics or rate of contraction, which are of primary important
in actuator applications, are reviewed.
Finally, some examples are given of actual devices constructed from
these contractile polymers.
2 Volume change
There are three competing forces acting on the gel
polymer network: the rubber elasticity, the polymer-polymer affinity and the
hydrogen ion pressure. These forces, collectively called the
osmotic pressure, determine the equilibrium state of the gel.
The competition between these forces determines the osmotic
pressure while the changing balance of these forces produces the volume
change.
Rubber elasticity tends to shrink the gel under tension and expand it under
compression. The elastic force is in equilibrium when the polymer ends are at
their root mean square distance. Although the equilibrium volume for
elasticity is independent of the external conditions,
its force is proportional to the absolute temperature.
Polymer-polymer affinity depends on the electrical attraction between the
polymer and the solvent. An attractive force between the polymer
and the solvent causes the absorb solvent molecules, while repulsive force
produces the opposite effect. This force does not depend on the
temperature, but on the solvent and volume of the gel.
Since polymer-polymer affinity is a short range force and depends only on
polymer-polymer contact, its effect is inversely proportional to the
square of the volume.
Hydrogen ion pressure is the force exerted by the motion of the hydrogen
ions H+ within the gel network. Hydrogen ions enter the gel attracted
by the negative charges on the polymer chain while their random motions tend to
expand the gel much as a gas exerts pressure within a contained volume.
The hydrogen ion pressure depends on the ionization of the
polymer, as well as, both temperature and volume. The force is linearly proportional
to the absolute temperature and inversely proportional to the square of the volume.
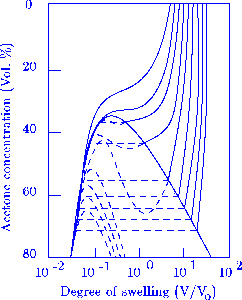
Figure 1
Volume changes in acrylamide gels are shown as a function of acetone concentration
and degree of hydrolysis.
3 Gel Preparation
Gels can be formed by condensation polymerization, where bifunctional and polyfunctional
units are combined, or by cross-linking polymers from bifunctional monomers.
Cross linkages can form from covalent bonds or through weak forces such as hydrogen
bonds, Van Der Waals forces or hydrophobic or ionic interactions. As an example,
consider the preparation of standard polyacrylamide gels (Tanaka 1981). Acrylamide
and bisacrylamide are dissolved in water together with polymerization initiators,
ammonium persulfate (AP) and tetramethylethylene diamine (TEMED). TEMED reacts
with AP leaving an unpaired valence electron which combines with acrylamide or
bisacrylamide transferring the unpaired electron to the acrylamide molecule, figure 2.
This procedure continues, forming an indefinitely large polymer network. After
washing, the gel is hydrolyized in a basic solution, converting the aminocarbonyl
side chains into carboxyl groups. The degree of hydrolyization determines the
percentage of carboxyl groups which greatly affects the volume phase transitions of
the gel (Hirokawa 1985, Nicoli 1983, Tanaka 1987a)
There are many other gels, all of which are characterized by their unique
side chains. The macroscopic structure of these gels and their preparation are basically
the same as the polyacrylamide. The preparation procedure for some of these gels
are outlined in appendix 1.
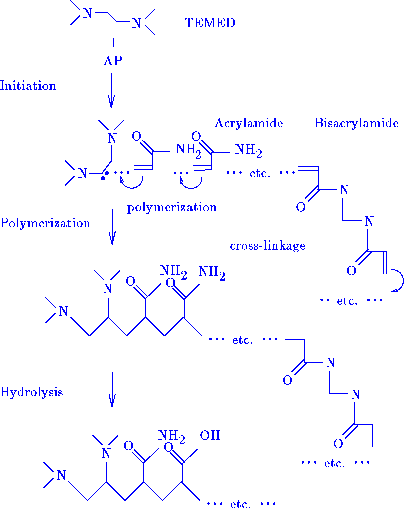
Figure 2
Activation of TEMED by AP yields an unpaired valence
electron which combines with
acrylamide or bisacrylamide forming an indefinitely large polymer network.
Hydrolysis in a basic solution converts aminocarbonyl groups into carboxyl
groups, which affects the volume phase transitions. (Tanaka 1981)
4 Kinetics
The kinetics or rate of swelling (or contraction) is an extremely
important characteristic in the application of gels to mechanical
actuation. The gel kinetics is a diffusion limited process and is
therefore proportional to the square of the dominant linear dimension
of the gel. Thus for a gel fiber, the contraction rate t is
equal to a contraction rate constant c times the square of the
diameter d^2, tc = cd^2. For polyacrylamide, c
is approximately 2 x 10^9 s/m^2. Therefore
polyacrylamide gels with a diameter of 1 cm take about 2.5 days to
contract, while micron diameter fibers take milliseconds. An
artificial muscle formed by bundling 25um diameter PAN-PPY
fibers was constructed and demonstrated contraction rates on the order
of a second (Chiarelli 1989).
In spite of its important for actuator design,
gel kinetics has only recently been investigated (Matsuo 1988a, 1988b, Tanaka 1987a).
Initially it
was thought that volume change was governed by the diffusion of individual
water molecules through the network, but it was latter shown
to be a collective diffusion process of the entire polymer network.
The local motion of the polymer network is given by a diffusion equation D = K/f,
where D is the diffusion coefficient,
K the elastic modulus of the network and f the frictional coefficient between the
network and the liquid (Chiarelli 1988).
The collective diffusion explains both the macroscopic swelling rates and the
dynamics of local density fluctuations.
Application
Though the phenomena of volume change within polymer network and the
chemical driven contractile forces they produce has been known for years
(Kuhn 1950, Flory 1956, Hamlem 1965), only recently have there been
any attempts to apply these materials to robotics.
A parallel jaw gripper driven by antagonistic
polyvinylachohol contractile elements powered by changes in acetone
concentration has been constructed (Caldwell 1989), along with
an artificial urethral sphincter, (Chiarelli 1989).
For the most part, however, these devices are still simple prototypes
stages.
The first gels were very slow, reaching an equilibrium volume anywhere
from one half an hour to a number of days.
Most gels were also weak, unable to support significant
loads. However, recent research has focused on thin films and bundles
of small fibers whose polymer networks are
aligned with the strain axis, allowing faster contraction rates and
higher strength.
Other attempts include superimposing a cross-linked a network onto a fiber
by projecting UV light through a mask. This
should yield a strong polymer and an effectively smaller dominant linear
dimension, producing a gel with both high strength and rapid contraction
(Zhang 1990).
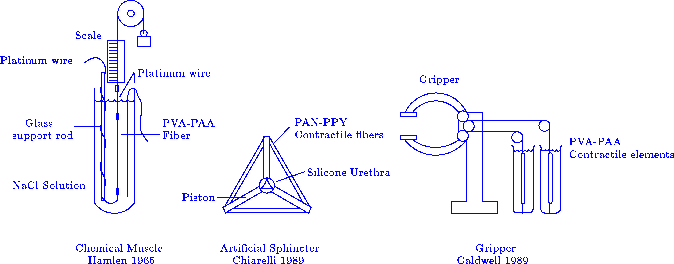
Figure 3
A force generating element, a robot gripper and artificial urethral sphincter
are some of the mechanical devices constructed from contractile polymer gel.
Conclusion
Despite some success in contractile gel
actuator development, there are a number of
fundamental questions which must be answered before consideration gels
in robotic devices. First, there are material questions
such as the elastic modulus, tensile strength,
stress-strain relations (Chiarelli 1990),
fatigue life, and thermal and electrical
conductivity. Second, there are thermodynamic issues such as
efficiency, power and force densities, and power limits. Third, there
are basic engineering concerns such as power supply and delivery,
device construction (De Rossi 1988),
manufacturing, power transmission, dynamic modelling
(Genuini 1990, Morasso 1990),
control, integration and packaging. Finally, there are other concerns
such as the coordination and integration of multiple actuators, material
and manufacturing costs and the toxicity of both the gels and their precursors. Many
promising technical advances (e.g. ceramic superconductors) have been limited
by basic physical or engineering deficiencies. However, given
limitations, there may be technical niches were these devices
may provide practical results. In any case without a thorough investigation
of the application of contractile gels to actuator design,
the extent or limit of this technology
is still unknown.
A Appendix. Gel Preparation
- N-Isopropylacrylamide (N-IPA) (Matsuo 1988)
- Preparation of paraffin oil
Wash paraffin oil with alkaline (1N NaOH) and acid (1N HCl) to remove
contaminants. Flush with large amounts of water. Pass paraffin oil
through a column packed with molecular sieves to remove water.
- Purify N-IPA
Recrystallize N-IPA with toluene, filter by
aspiration and dry under a vacuum. Store in a colored bottle to
avoid light exposure.
- Monomer solution
Combine 700mM N-isopropylacrylamide (N-IPA),
8.6mM N,N'-methylenebisacrylamide (BIS) and
0-128mM sodium acrylate (prepared from acrylic acid and sodium bicarbonate).
- Polymerization
Add 48 ul tetramethylethylenediamine (TEMED) and
0.2 ml of ammonium persulfate to 10 ml monomer solution
- Gelation
Inject 1 ml per-gel solution into 50 ml paraffin oil, degas,
saturate with nitrogen, and disperse into submillimeter droplets. Agitate
for one hour at 20C, then pour solution in 1000 ml water. Use
petroleum ether to removal paraffin oil. Wash gel beads several times.
- Polyacrylonitrile-polypyrrole (PAN-PPY) (Chiarelli 1989)
- Anneal PAN
Anneal PAN (Mitsubishi Rayon Co.)
at 220C for 5 hours and then boil
in 1N NaOH for 30 min. to produce a partially ionized carboxylic structure
- Combine with PPY
Immerse fibers for 24 hrs. in an aqueous solution of
ferric chloride (40% by weight). Add HCL to pH = 0.5. Polymerize PPY in PAN
by gas state technique (Ojio and Miyata). Place in flask under saturated
atmosphere of water and PPY at 10Cfor 5 to 20 hours.
- Polyvinylalcohol-polyacrylic acid (PVA-PAA) (Chiarelli 1988)
- Mix components
Dissolve 80% PVA with a degree of hydrolisis of 98% and
molecular weight 10k (AnalytiCals, Carlo Erba, Milano, Italy)
with 20% PAA molecular weight 250k
(Aldrich Chemical Co., Milwaukee, WI, USA) in bidistilled water and
stir for 20 min. at 60C.
- Dehydrate
Dehydrate solution in at 40C under mild vacuum
- Cross-link
Thermally cross-link polymer at 130C for 45 min., then
equilibrate with bidistilled water.
References
- Caldwell, D. and Taylor, P., Hull University, 1990.
- Chiarelli, P. and De Rossi, D., ``Determination of Mechanical Parameters
related to the Kinetics of Swelling in an Electrically Activated Contractile
Gel,'' Progress in Colloid and Polymer Science, Vol. 48, 1988.
- ---, et. al., ``Progress in the Design of an Artificial
Urethral Sphincter,'' Proc. of the 3rd Vienna International
Workshop on Functional Electrostimulation, Vienna Austria, Sept. 1989.
- ---, et. al., ``Dynamics of a Hydrogel Strip,''
In Press, Biorheology, 1990.
- De Rossi, D., et. al., ``Contractile Behavior of Electrically
Activated Mechanochemical Polymer Actuators,'' Transactions of the
American Society of Artificial Internal Organs XXXII, p. 157-162, 1986.
- ---, et. al., ``Analogs of Biological Tissues for Mechanoelectrical
Transduction: Tactile Sensors and Muscle-Like Actuators,''
NATO ASI Series, Vol. F43, Sensors and Sensory Systems, 1988.
- Flory, P. J., ``Role of Crystallization in Polymers and Proteins,''
Science, Vol. 124, p. 53-60, 1956.
- Genuini, G., et. al., ``Psuedomuscular Linear Actuators: Modelling and
Simulation Experiences in the Motion of Articulated Chains,'' In Press,
NATO ACI Science, 1990.
- Hamlem, R. P., Kent, C. E., and Shafer, S. N., ``Electrolytically Activated Contractile
Polymers,'' Nature, Vol. 206, p. 1149-1150, 1965.
- Hirokawa, Y., Tanaka, T. and Sato, E., ``Phase Transition of Positively
Ionized Gels'', Macromolecules, Vol. 18, p. 2782, Dec. 1985.
- Kuhn, W., et. al., ``Reversible Dilatation and Contraction by Changing the State of
Ionization of High-polymer Acid Networks,'' Nature, Vol. 165, p. 514-516, 1950.
- Li, Y. and Tanaka, T., ``Study of the Universality Class of the Gel Network System,''
Journal of Chemical Physics, Vol. 90, No. 9, p.5161-5166, 1989.
- Morasso, P., et. al., ``Generation of Command Synergies for
Anthropomorphic Robots,'' Proc. IEEE of the Conference on Robotics and
Automation, 1990.
- Matsuo, Eriko Sato and Tanaka, Toyoichi, ``Kinetics of discontinuous volume-phase
transition of gels'', Journal of Chemical Physics, Vol. 89, No. 3, 1988.
- ---, ``Kinetic and Light Scattering Studies of Phase Transitions
of N-Isopropylacrylamide Gels,'' MIT PhD Thesis, 1988.
- Nicoli, D., et. al., ``Chemical Modification of Acrylamide Gels: Verification of
the Role of Ionization in Phase Transitions,'' Macromolecules, Vol. 16,
p. 887-891, 1983.
- Tanaka, T., ``Gels'', Scientific American, p. 124-138, January 1981.
- ---, Nishio, I., Sun, S.T., and Ueno-Nishio, S., ``Collapse of Gels under
an Electric Field'', Science, Vol. 218, p. 467, 1982.
- ---, et. al., ``Mechanical instability of gels at the phase transition'',
Nature, Vol. 325, No. 6107, pp. 796-798, February 26, 1987.
- ---, ``Gels,'' in Structure and Dynamics of Biopolymers, edited by
Nicolini, C., Martinus Nijhoff Publishers, Boston, p. 237-257, 1987.
- Zhang, Yong-Qing, personally conversations, 1990.
Literature
- Candau, S. J., et. al., ``Intensity of Light Scattered from Polymeric Gels:
Influence of the Structure of the Networks,'' Journal of Chemical Physics,
Vol. 70, p. 4694, 1979.
- Chiarelli, P. and De Rossi, D., ``Determination of Mechanical Parameters
related to the Kinetics of Swelling in an Electrically Activated Contractile
Gel,'' Progress in Colloid and Polymer Science, Vol. 48, 1988.
- ---, et. al., ``Progress in the Design of an Artificial
Urethral Sphincter,'' Proc. of the 3rd Vienna International
Workshop on Functional Electrostimulation, Vienna Austria, Sept. 1989.
- ---, et. al., ``Dynamics of a Hydrogel Strip,''
In Press, Biorheology, 1990.
- ---, Umezawa, K, and De Rossi, D., ``A Polymer Composite showing
Electrocontractile Response,'' submitted to Journal of Polymer Science
Polymer Letters Edition
- De Rossi, D., et. al., ``Electrically Induced Contractile Phenomena in Charged
Polymer Networks Preliminary Study on the Feasibility of the Muscle-like Structures,''
Transactions of the American Society of Artificial Internal Organs XXXI,
p. 60-65, 1985.
- ---, et. al., ``Contractile Behavior of Electrically
Activated Mechanochemical Polymer Actuators,'' Transactions of the
American Society of Artificial Internal Organs XXXII, p. 157-162, 1986.
- --- and Chiarelli, P., ``Determination of Mechanical Parameters
related to the Kinetics of Swelling in an Electrochemically Actuated
Contractile Gel,'' Abstract 5th. International Seminar on Polymer
Physics, High Tatras, 1987.
- ---, et. al., ``Analogs of Biological Tissues for Mechanoelectrical
Transduction: Tactile Sensors and Muscle-Like Actuators,''
NATO ASI Series, Vol. F43, Sensors and Sensory Systems, 1988.
- Eisemberg, S. R. and Grodzinsky, A. J., ``Swelling of Articulated Cartilage and
other Connective Tissues: Electromechanochemical Forces,'' Journal of
Orhopaedic Research, Vol. 3, p. 148-159, 1985.
- Flory, P. J., ``Role of Crystallization in Polymers and Proteins,''
Science, Vol. 124, p. 53-60, 1956.
- Genuini, G., et. al., ``Psuedomuscular Linear Actuators: Modelling and
Simulation Experiences in the Motion of Articulated Chains,'' In Press,
NATO ACI Science, 1990.
- Grodzincky, A. J., and Shoenfeld, N. A., ``Tensile Forces induced in
Collagen by means of Electromechanochemical Transductive Coupling,''
Polymer, Vol. 18, p. 435-443, 1977.
- ---, ``Electromechanical and Physiochemical Properties of
Connective Tissue,'' CRC Critical Review in Biomedical Engineering,
Vol. 9., No. 2., p. 133-199. 1983.
- Hamlem, R. P., Kent, C. E., and Shafer, S. N., ``Electrolytically Activated Contractile
Polymers,'' Nature, Vol. 206, p. 1149-1150, 1965.
- Hasa, J., Ilavsky, M. and Dusek, K., ``Deformation, Swelling, and Potentiometric
Behavior of Ionized Poly(methacrylic Acid) Gels. I Theory,'' Journal of
Polymer Science: Polymer Physics Edition, Vol. 13, p. 253-262, 1975.
- Hirokawa, Y. and Tanaka, T., ``Volume Phase Transition in a Non-Ionic Gel,''
in Physics and Chemistry of Dorons Media, edited by Johnson, D. L. and
Sen, P. N., American Institute of Physics, New Your, 1984.
- ---, Tanaka, T. and Sato, E., ``Phase Transition of Positively
Ionized Gels'', Macromolecules, Vol. 18, p. 2782, Dec. 1985.
- ---, et. al., ``Mechanical Instability of Gels Undergoing Large Swelling,''
in Physical Optics of Dynamnical Phenomena and Processes on Macromolecular Systems,
edited by Sedlacek, deGruyter W. and Company, New York, p. 197, 1985.
- Hirotsu, S., Hirokawa, Y. and Tanaka, T., ``Volume-phase transitions of ionized
N-isopropylacrylamide gels,'' Journal of Chemical Physics, Vol. 87., No. 2,
p. 1392-1395, 1987.
- Hochber, A., Tanaka, T., and Nicoli C., ``Spinodal Line and Critical Point of an
Acrylamide Gel,'' Physical Review Letters, Vol. 43, p. 217, 1979.
- Ishimoto, C. and Tanaka, T., ``Critical Behavior of a Binary Mixture of Protein/
Salt-Water,'' Physical Review Letters, Vol. 39, p. 474, 1977.
- Itoh, Y., et. al., ``Contraction/Elongation Mechanism of Acrylonitrile Gel Fibers,''
Polymer Preprints - Japan (English Edition), Vol. 36, Nos. 5-10, p. E184, 1987.
- Johnson, D. L., ``Elastodynamics of Gels,'' Journal of Chemical Physics,
Vol. 77, p. 1531-1540, 1982.
- Katayama, S., Hirokawa Y. and Tanaka, T., ``Reentrant Phase Transitions of
Acrylamide Derived Gels,'' Macromolecules, 1984.
- Katchalsky, A., et. al., Chapter ``Elementary Mechanochemical Processes,''
Size and Shape Changes of Contractile Polymers, Wassermann, A. ed.,
Pergamon Press, New York, 1960.
- --- and Oplatka, A., ``Mechano-chemical Conversion,'' in
Loewenstein, W. R., (ed), Principles of Receptor Physiology, New York,
Springer-Verlag, p. 1-17, 1971.
- ---, et. al., ``Reversible Dilatation and Contraction by Changing the State
of Ionization of High-polymer Networks,'' Nature, Vol. 165, p. 514-516.
- --- and Hargitay, B., ``Muskelahniche Arbeistleisstung Kustlicher
Hochpolymer Stoffe,'' Z. Elektrochem, Vol. 55, p. 490-502.
- ---, Remel, A. and Walters, D. H., ``Conversion of Chemical into Mechanical
Energy by Homogeneous and Cross-striated Polymeric Systems,'' in Wassermann, A.,
(ed.), Size and Shape Changes of Contractile Polymers, Pergamon Press, New York,
p. 41-77, 1960.
- Kuhn, W., et. al., ``Reversible Dilatation and Contraction by Changing the State of
Ionization of High-polymer Acid Networks,'' Nature, Vol. 165, p. 514-516, 1950.
- Lazzeri, L., ``Analisi di Fenomeni Contracttili in Gel di Plielecttroliti in
Presenza di campi Electtrici di Bassa Intensita,'' Testi di Laurea in
Fisica, Universita degli Studi di Pisa, 1987.
- Li, Yong, ``Structure and Critical Behavior of Polymer Gels,'' PhD thesis MIT, 1989.
- --- and Tanaka, T., ``Study of the Universality Class of the Gel Network System,''
Journal of Chemical Physics, Vol. 90, No. 9, p.5161-5166, 1989.
- Mackie, J. S. and Meares, P., ``The Diffusion of Electrolytes in a
Cation-exchange Resin Membrane,'' Proc. Roy. Soc. A., p. 232-498, 1955.
- Mandelkern, L., ``Contractile Processes in Fibrous Macromolecules,'' Ann.
Rev. Phys. Chem., Vol. 15, p.421-448.
- Michaeli, I. and Katkalsky, A., ``Potentiometric Titration of Polyelectolyte gels,''
Journal of Polymer Science, Vol. 23, p. 683-696, 1957.
- Morasso, P., et. al., ``Generation of Command Synergies for
Anthropomorphic Robots,'' Proc. IEEE of the Conference on Robotics and
Automation, 1990.
- Matsuo, Eriko Sato and Tanaka, Toyoichi, ``Kinetics of discontinuous volume-phase
transition of gels'', Journal of Chemical Physics, Vol. 89, No. 3, 1988.
- ---, ``Kinetic and Light Scattering Studies of Phase Transitions
of N-Isopropylacrylamide Gels,'' MIT PhD Thesis, 1988.
- Nagasawa, M., ``Molecular Conformation and Dynamics of Macromolecules in Condensed Systems,''
Studies in Polymer Science, Elsevier, New York, 1988.
- Nakatani, Y., Ourisson, G. and Tanaka, T., ``Osmotic Swelling of Phospholipid Vesicles,''
Biophysics Biochemical Research Communications, Vol. 110, p. 1320, 1983.
- Nicoli, D., et. al., ``Chemical Modification of Acrylamide Gels: Verification of
the Role of Ionization in Phase Transitions,'' Macromolecules, Vol. 16,
p. 887-891, 1983.
- Nishio, Izumi, et. al., ``Critical Density Fluctuations within a Single Polymer Chain,''
Nature, Vol. 300, No. 5889, p. 243-244, 1982
- Nussbaum, J. H. and Grodzinsky, A. J., ``Proton Diffusion Reaction in a Protein
Polyelectrolyte Membrane and the Kinetics of Electromechanical Forces,''
Journal of Membrane Science, Vol. 8, p. 193-219, 1981.
- Ohmine, I. and Tanaka, T., ``Salt Effects on the Phase Transitions of Polymer Gels,''
Journal of Chemical Physics, Vol. 77, p. 5725, 1982.
- Ojio, T. and Miyata, T., ``Highly Transparent and Conducting Polypyrrole-Poly(vinyl alcohol)
Composite Films Prepared by Gas State Polymeriztion,'' Polymer Journal, Vol. 18, p. 95-98.
- Osada, T. and Hasebe, M., ``Electrically Actuated Mechanochemical Devices using
Polyelectrolyte Gels,'' Chemistry Letters,'', p. 1285-1288, 1985.
- Ricka, J. and Tanaka, T., ``Swelling of Ionic Gels: Quantitative Performance of the
Donnan Theory,'' Macromolecules, 1984.
- Ricka, J. and Tanaka, T., ``Phase Transition in Ionic Gels Induced by Copper Complexion,''
Macromolecules, 1984.
- Sun, S. T., et. al., ``The Coil-Globure Transition: Radius of Gyration of Polystyrene in
Cyclochexane,'' Journal of Chemical Physics, vol. 73, p. 5971, 1980.
- Suzuki, M., et. al., ``An Artificial Muscle by Polyvinyl Alcohol Hydrogel Composites,''
Proc. of IUPAC-CHEMRAWN VI, Tokyo, 1987.
- Swislow, G., et. al., ``Coil-Globule Phase Transition in a Single Polystyrene Chain in
Cyclohexane,'' Nature, Vol. 281, p. 208, 1979.
- Tanaka, T., Soda, K. and Wada, A., ``Dynamical Aspects of Helix-Coil Transitions in
Biopolymers I,'' Journal of Chemical Physics, Vol. 58, p. 5707, 1973.
- --- and Suzuki, M., ``Spin Correlations and Cummulants of Ising Chains with
Applications to Helix-Coil Transitions,'' Journal of Chemical Physics, Vol. 59,
p. 3795, 1973.
- --- --- and Wada, ``Dynamical Aspects of Helix-Coil Transitions in
Biopolymers II,'' Journal of Chemical Physics, Vol. 59, p. 3799, 1973.
- ---, Hocker, L. O. and Benedek, G. B., ``Spectrum of Light Scattered from a
Viscoelastic Gel,'' Journal of Chemical Physics, Vol. 59, p. 5151-5159, 1973.
- ---, Ishiwata, S and Ishimoto, C., ``Critical Behavior of Density Fluctuations
in Gels,'' Physical Review Letters, Vol. 38, p. 771, 1977.
- ---, ``Dynamics of Critical Concentration Fluctuations in Gels,''
Physics Review, V. A17, p. 763, 1978
- ---, ``Collapse of Gels and the Critical Endpoint,'' Physical Review Letters,
Vol. 40, p. 820, 1978.
- --- and Fillmore, D. J., ``Kinetics of Swelling of Gels,'' Journal of Chemical
Physics, Vol. 70, p. 1214, 1979.
- ---, Swislow, G. and Ohmine, I., ``Phase Separation and Gelation in Gelatin Gels,''
Physical Review Letters, Vol. 42, p. 1556, 1979
- ---, ``Light Scattering from Gels and a Single Polymer Chain near Phase Transition,''
in Light Scattering in Solids, edited by Birman, J. L., Cummins, H. Z. and
Rebane, K. K., Plenum Press, p. 29-37, 1979.
- ---, ``Phase Transition in Gels and a Single Polymer,'' Polymer, Vol. 20, p. 1404,
1979.
- ---, et. al., ``Phase Transition in Ionic Gels,'' Physical Review Letters, Vol. 45,
p. 1636, 1980.
- ---, ``Gels'', Scientific American, p. 124-138, January 1981.
- ---, Nishio, I., Sun, S.T., and Ueno-Nishio, S., ``Collapse of Gels under
an Electric Field'', Science, Vol. 218, p. 467, 1982.
- ---, ``Light Scattering from Polymer Gels,'' in Dynamics Light Scattering,
edited by Pecora, R, Plenum Press, p. 347, 1985.
- ---, et. al., ``Mechanical instability of gels at the phase transition'',
Nature, Vol. 325, No. 6107, pp. 796-798, February 26, 1987.
- ---, ``Gels,'' in Structure and Dynamics of Biopolymers, edited by
Nicolini, C., Martinus Nijhoff Publishers, Boston, p. 237-257, 1987.
- Wada, A., Tanaka, T. and Kihara, H., ``Dielectric Dispersion of the Alpha-Helix
at the Transition Region to Random Coil,'' Biopolymers, Vol. 11, p. 587-, 1972.



