
Next: Methods
Up: Design Considerations for a
Previous: Design Considerations for a
Diabetic macular edema and age-related macular degeneration (AMD) are the two
major causes of visual loss in developed countries. While laser therapy for
these and other diseases has prevented loss of visual function in many
individuals, disease progression and visual loss following suboptimal
treatment is common. For AMD, there is unambiguous evidence that incomplete
laser photocoagulation of the border of a choroidal neovascular lesion is
associated with an increased risk for further visual loss, while treatment
beyond the borders unnecessarily destroys viable, central photoreceptors,
further degrading visual function.
As a concrete example, in eyes with juxtafoveal choroidal
neovascularization (CNV) secondary to ocular histoplasmosis, only
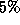 of eyes with laser treatment that covered the foveal side of the
lesion with a narrow
of eyes with laser treatment that covered the foveal side of the
lesion with a narrow 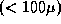 treatment border suffered severe
visual acuity loss, while
treatment border suffered severe
visual acuity loss, while 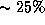 of eyes with either some of the
foveal side untreated, or a wide border of treatment on the foveal
side suffered severe visual loss (MPS, 1995). Similar results have
been reported for AMD. Building on the recommendations proposed in
Macular Photocoagulation Studies, clinicians generally attempt to
correlate angiographic data with biomicroscopic images using crude,
time-consuming, potentially error-prone methods. In a recent practical
review (Neely, 1996), the author suggests that ``... to assist you
in treatment (of neovascular AMD), project an early frame of the
fluorescein angiogram onto a viewing screen. Use the retinal vessels
overlying the CNV lesion as landmarks. I suggest tracing an image of
the CNV lesion and overlying vessels onto a sheet of onion skin
paper. It takes a little extra time, but I find it helps to clarify
the treatment area.'' Accordingly, precise identification of the
treatment border during laser therapy by correlating the
biomicroscopic image with fluorescein angiographic data (where the
lesion extent is better delineated, see for example Figure
1) should be beneficial for maximizing post-treatment
visual function. Diagnosis and treatment relies on synthesizing
clinical data derived from fundus biomicroscopy with angiographic
data, but methods for correlating these data, and for direct guidance
of laser therapy, are not well-developed.
of eyes with either some of the
foveal side untreated, or a wide border of treatment on the foveal
side suffered severe visual loss (MPS, 1995). Similar results have
been reported for AMD. Building on the recommendations proposed in
Macular Photocoagulation Studies, clinicians generally attempt to
correlate angiographic data with biomicroscopic images using crude,
time-consuming, potentially error-prone methods. In a recent practical
review (Neely, 1996), the author suggests that ``... to assist you
in treatment (of neovascular AMD), project an early frame of the
fluorescein angiogram onto a viewing screen. Use the retinal vessels
overlying the CNV lesion as landmarks. I suggest tracing an image of
the CNV lesion and overlying vessels onto a sheet of onion skin
paper. It takes a little extra time, but I find it helps to clarify
the treatment area.'' Accordingly, precise identification of the
treatment border during laser therapy by correlating the
biomicroscopic image with fluorescein angiographic data (where the
lesion extent is better delineated, see for example Figure
1) should be beneficial for maximizing post-treatment
visual function. Diagnosis and treatment relies on synthesizing
clinical data derived from fundus biomicroscopy with angiographic
data, but methods for correlating these data, and for direct guidance
of laser therapy, are not well-developed.
We are exploring techniques to overlay angiographic data on the real-time
biomicroscopic slitlamp fundus image in order to guide treatment for eye
disease (for example, to better define and visualize the edges of choroidal
neovascular membrane, and improve identification of focal areas of leakage in
diabetic macular edema). The biomicroscopic fundus image will be ``augmented''
in real time with available angiographic data. Text display and a ``virtual
pointer'' will be incorporated into the augmented reality display to
facilitate teaching, telemedicine, and real-time measurement and image
analysis. Moreover, image superposition will allow for direct comparison with
previous images to judge disease progression (for example, to judge
progression or stability of AMD or cytomegalovirus retinitis---a common,
blinding disease afflicting patients with acquired immunodeficiency syndrome)
and allow for real-time identification of prior treatment areas. This
technology is straightforwardly extended to an indirect-ophthalmoscope-based
or operating-microscope-based system to facilitate correlative, teaching, and
telemedicine applications in these environments.
In this report, we describe considerations for the design and implementation
of an ophthalmic augmented reality environment and report on our preliminary
studies in model systems.
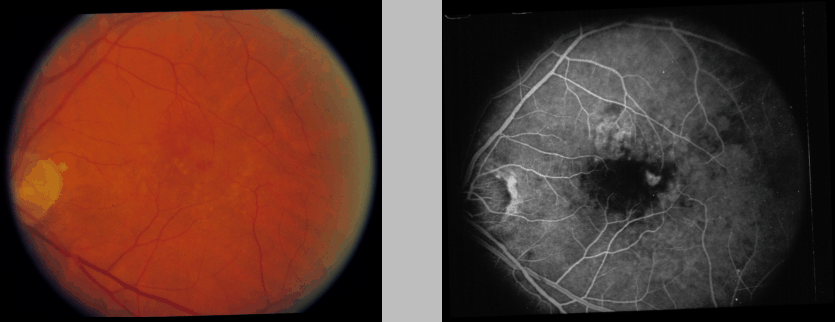
Figure: Monochromatic photographic
(left) and fluorescein angiographic (right) image of an eye with
age-related macular degeneration. The optic nerve is at the far left
of each photograph, with the fovea located centrally. Note that the
angiogram conveys additional information regarding areas of leaky
blood vessels.


Next: Methods
Up: Design Considerations for a
Previous: Design Considerations for a
Michael E. Leventon
Tue Dec 17 12:28:43 EST 1996
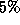 of eyes with laser treatment that covered the foveal side of the
lesion with a narrow
of eyes with laser treatment that covered the foveal side of the
lesion with a narrow 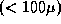 treatment border suffered severe
visual acuity loss, while
treatment border suffered severe
visual acuity loss, while 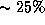 of eyes with either some of the
foveal side untreated, or a wide border of treatment on the foveal
side suffered severe visual loss (MPS, 1995). Similar results have
been reported for AMD. Building on the recommendations proposed in
Macular Photocoagulation Studies, clinicians generally attempt to
correlate angiographic data with biomicroscopic images using crude,
time-consuming, potentially error-prone methods. In a recent practical
review (Neely, 1996), the author suggests that ``... to assist you
in treatment (of neovascular AMD), project an early frame of the
fluorescein angiogram onto a viewing screen. Use the retinal vessels
overlying the CNV lesion as landmarks. I suggest tracing an image of
the CNV lesion and overlying vessels onto a sheet of onion skin
paper. It takes a little extra time, but I find it helps to clarify
the treatment area.'' Accordingly, precise identification of the
treatment border during laser therapy by correlating the
biomicroscopic image with fluorescein angiographic data (where the
lesion extent is better delineated, see for example Figure
1) should be beneficial for maximizing post-treatment
visual function. Diagnosis and treatment relies on synthesizing
clinical data derived from fundus biomicroscopy with angiographic
data, but methods for correlating these data, and for direct guidance
of laser therapy, are not well-developed.
of eyes with either some of the
foveal side untreated, or a wide border of treatment on the foveal
side suffered severe visual loss (MPS, 1995). Similar results have
been reported for AMD. Building on the recommendations proposed in
Macular Photocoagulation Studies, clinicians generally attempt to
correlate angiographic data with biomicroscopic images using crude,
time-consuming, potentially error-prone methods. In a recent practical
review (Neely, 1996), the author suggests that ``... to assist you
in treatment (of neovascular AMD), project an early frame of the
fluorescein angiogram onto a viewing screen. Use the retinal vessels
overlying the CNV lesion as landmarks. I suggest tracing an image of
the CNV lesion and overlying vessels onto a sheet of onion skin
paper. It takes a little extra time, but I find it helps to clarify
the treatment area.'' Accordingly, precise identification of the
treatment border during laser therapy by correlating the
biomicroscopic image with fluorescein angiographic data (where the
lesion extent is better delineated, see for example Figure
1) should be beneficial for maximizing post-treatment
visual function. Diagnosis and treatment relies on synthesizing
clinical data derived from fundus biomicroscopy with angiographic
data, but methods for correlating these data, and for direct guidance
of laser therapy, are not well-developed.