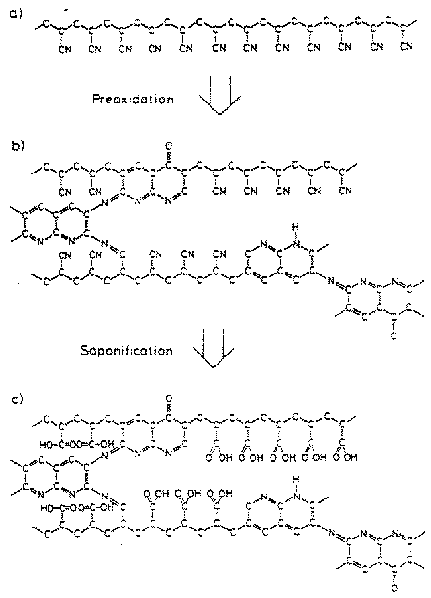
Figure 1. Schematic illustration of the structure of PAN fibers. (figure from [6.]).
7 U.R.G.
Muscle Project
Aritificial Intelligent Laboratory
Massachusetts Institute of Technology
Muscle possesses certain characteristics and qualities which are essential to its function. Some of these include strength, size, response time, and the ability to contract and expand. In the pursuit of a suitable replacement for muscle these factors must be taken into consideration. Although many substances might make adequate alternatives for muscle, polymer gels are currently being used in the development of an artificial muscle with strength and speed equal to that of a human muscle.
A polymer is a long chain of molecules. A large network of polymers can be formed when several of these chains become chemically bonded to each other through a process known as cross-linking. When this network of cross-linked polymers is suspended in a solvent, it becomes a polymer gel. Currently, polymers are playing many different roles, including insulation, structural support, and adhesion. [Mitwalli 1994] Polymer gels can be designed to exhibit large, abrupt, discontinuous changes in volume in response to a variety of different stimuli. These volume changes are reversible and often as large as 1000 fold.1 Common forms of stimulation for these changes include temperature, light, electric fields, pH, and various solvents. [Umemoto 1991] Because of these unique attributes, attention has recently turned towards the application of polymer gels to the realm of synthetic muscle with much success.
In one model, polymer gels have been designed to change volume in response to changes in pH. The fibers exhibit stronger contraction force than the human muscle and quick response times. Commercially available poly (acrylonitrile) (PAN) gel fibers are presently being used in this project. PAN fibers consist of long carbon chains with nitrile groups attached. When heated for long periods of time these chains bond to each other in a process known as cross-linking. Boiling this network of fibers in NaOH solution causes the remaining unlinked nitrile groups to convert to carboxylic acids as seen in Figure 1. These carboxylic acids are essential to the behavior of the polymer gel. In an acidic solution these carboxyl groups are not charged and the gel forms a compact structure. In the presence of a base, though, the carboxyl groups are negatively charged and the electrostatic interactions between these charged groups cause the gel to swell. This allows for the expansion and contraction of the gel which simulates the behavior of muscle. However, because of the presence of basic pyridyl and carboxyl groups, it is necessary to add a significant amount of acid or base to bring about these behavioral changes. Over a large pH range, the carboxylic acid moieties remain anionic, while the pyridyl groups are still protonated. This buffering action became the focus of these experiments because it deters from the speed and efficiency that the synthetic muscle could possess.
Using knowledge of the principles of polyelectrolyte networks and the relations between the polymer's structure and properties, it may be possible to modify the gel to obtain the desired characteristics. There are several approaches for modifying the pre-oxidated or saponified PAN fibers which should allow the gel to respond over narrower pH ranges and, therefore, increase the speed and efficiency of the gel system. In these experiments it was attempted to modify the currently used PAN gel fibers in a variety of ways to limit these detrimental factors: first by altering nitrile groups and secondly by converting carboxyl groups as shown in reactions 1 and 2.

Figure 1. Schematic illustration of the structure of PAN fibers. (figure from [6.]).
2.1.1 Anhydrous diethyl ether (30 mL) was added to two segments of the cross-linked fiber (0.009 g and 0.011 g). Over the course of 10 minutes, a 1 M solution of LiAlH4 in anhydrous diethyl ether (10 mL) was added through an addition funnel. After 45 minutes, the polymer was removed and rinsed with distilled water.
2.1.2. Tetrahydrofuran (THF) (50 mL) was added to a segment of the cross-linked fiber (0.010 g). The solution was heated to boiling (66 C) and refluxed throughout the procedure. Over the course of 10 minutes, a 1 M solution of LiAlH4 in anhydrous diethyl ether (10 mL) was added. After 30 minutes additional THF (10 mL) and 1M LiAlH4 (5 mL) were added. The mixture was boiled for one hour total and then the polymer was removed and rinsed with distilled water.
2.1.3 THF (100 mL) was added to a segment of an uncross-linked fiber (0.012 g), a cross-linked fiber (0.010 g), and a cross-linked fiber (0.009 g) which had been pulled apart to allow for deeper penetration of the other reactants. The solution was heated to boiling (66 C) and refluxed throughout the procedure. Over the course of 10 minutes, a 1 M solution of LiAlH4 in anhydrous diethyl ether (25 mL) was added. After one and a half hours additional 1M LiAlH4 (5 mL) was added. The mixture was boiled for two hours total and then the polymer was removed and rinsed with distilled water.
2.2.1 t-BuOH (100 mL) was heated to a liquid state and then added to the saponified fiber. The solution was heated and diphenyl phosphorylazide (DPPA) (5.5 g) and triethylamine (TEA) (2.1 g) were added via addition funnel. The reaction was allowed to reflux for 23 hours. The polymer was then removed, rinsed with distilled water, and placed in 1N NaOH aqueous solution. The solution was boiled for 30 minutes.
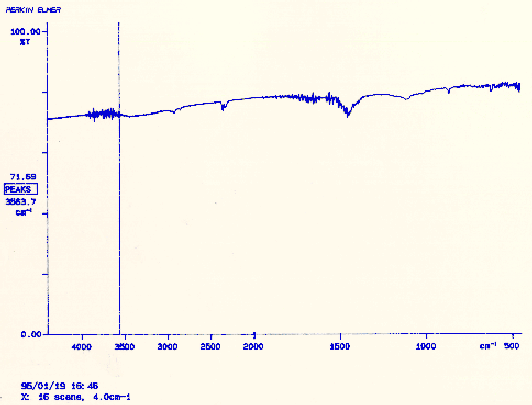
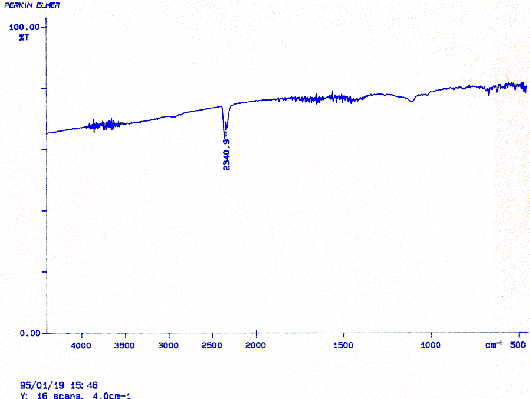
Figure 2.
The first attempt to modify the gel fiber concentrated on altering nitrile groups. The fiber used was cross-linked but had not been boiled in the NaOH solution, so there were no carboxyl groups present. By changing the nitrile groups to amines, the swelling of the gel would be reversed. In a basic solution the gel would be neutral and contracted. In an acid, though, the amines would become positively charged, causing the gel to swell. By altering the gel in this way it was anticipated that the range of pH over which there is no change in length would be reduced and the time of reaction shortened in the process. This type of reaction, however, proved to be more difficult than expected.
Reaction 1.1 was conducted at room temperature. The fiber did not gel implying that no reaction occurred. Looking more carefully at the reaction parameters revealed why this reaction was not successful. Performing these reactions on a soluble substance might have resulted in success, but this fiber is insoluble, thick, and inpenetrable. When the cross-linked polymer is allowed to sit in a 1N NaOH solution no reaction takes place, but when it is boiled the saponification occurs. The NaOH solution is made from distilled water and has a boiling point around 100 C. When the fiber is boiled in the NaOH solution the reactants possess more energy and are able to overcome the steric hindrance of the fiber, allowing the reaction to proceed. The conclusion after the first experiment was that there simply was not enough energy present to overcome this steric hindrance, preventing any reaction.
In order to increase the energy of the reactants, the reaction was carried out at a higher temperature. Because the boiling point of diethyl ether is so low, another solvent with a higher boiling point was necessary. THF has a boiling point of 66 C and is a good solvent for reactions involving LiAlH4, so it was chosen for reaction 1.2. The experiment was setup again using the new solvent and a refluxing tube to reduce the escape of any evaporated chemicals due to refluxing. Halfway through the reaction additional solvent and 1M LiAlH4 in diethyl ether were added to the reaction flask because some evaporation was taking place. The nitrogen flow was probably too high and some of the volatile chemicals were being carried out with it. This reaction also produced no gelling. It was theorized that the steric hindrance of the cross-linked polymer was still too great for the amount of energy that the reactants possessed. The fiber does not gel in the NaOH solution unless it is boiled for at least 30 minutes and THF has a lower boiling point than the NaOH solution, making this conclusion reasonable.
In an attempt to correct for this problem reaction 1.3 was repeated under similar conditions but with an extended period of boiling. This also proved unsuccessful. After each of these experiments, there was still plenty of reactive LiAlH4 present so the lack of any reaction could not have been due to an absence of reactants. The conclusion at this point was that an increase in the energy level was still needed to overcome the steric hindrance of the fiber. A pressure chamber would allow for a further increase in temperature. At this point the focus was switched to a different modification.
The new approach was an attempt to convert the carboxylic acids to amines. A gel that would swell due to net positive charge should still result, with contraction occurring in basic solution and the reverse occurring in acid. This approach was chosen because it required the presence of carboxyl groups found on the gelled polymers. Gelled fibers are not as compact as their ungelled counterparts. The inner parts of the fiber are, therefore, more exposed reducing steric hindrance and requiring less energy for the penetration of the fiber. Such a procedure requires the use of saponified fibers so the cross-linked polymers were first boiled in a NaOH solution. Since the protocol called for a long boiling period and previous refluxing under a nitrogen flow had led to some evaporation, it was decided that a CaCl2 drying tube should be used instead to prevent overnight boiling off of any reactants. In a previous study by Ninomiya, et. al.4, t-BuOH had produced the best results as a solvent so it was chosen for reaction 2.1. To complete the conversion from carboxylic acid to amine the fiber was boiled in a 1N NaOH solution. The degree of success of the procedure was then examined.
The fiber started in a gelled state and it still existed in this state at the end of the procedures. The sensitivity of the gel to acidic and basic solutions, however, revealed a problem. It was expected that the contraction/expansion behavior would reverse after the conversion. Because this did not occur, it suggested that no conversion had occurred. It was possible that a partial reaction had taken place but in order to better understand the precise situation IR analysis was performed. The fiber from reaction 2.1 and an unconverted one were analyzed. Because the polymer is insoluble in any solvent, IR analysis is difficult due to its lack of transparency. The polymer was prepared are previously described and tested for infrared transmittance. Comparison of the IR spectra for each fiber showed that no conversion had occurred. In the future, additional modification of reaction 2.1, by the changing of reactants or reaction conditions will be necessary to induce the conversion.
Although these results show failure they are still encouraging. If a conversion had occurred, and the gel still did not behave as expected, then proceeding along this line of investigation would not be productive. Since no reaction actually occurred in these experiments, there is still hope that further modification of the procedures could lead to a successful conversion. If conversion is successful then the desired results might be obtained. This would lead us one step closer in the quest to develop synthetic muscle.
Support for the research at the Artificial Intelligence Laboratory was provided by Sandia National Laboratory under contracts numbers AI-3367 and AA-9823, and support for research at Sandia National Laboratory was provided by the U.S. Department of Energy under contract number DE-AC04-94AL85000.
I would like to thank David Brock who supervised and advised throughout the project. Thanks is also given to Prof. Alexander Rich for use of his laboratory facilities, without which no progress could have been made. Paula Hammond, Steve Reid, and Andres Jaschke also offered invaluable advise and without the aid of Jim Simms , the IR analysis would not have been possible.
1. Brock, D., "Review of Artificial Muscle based on Contractile Polymers," A.I.Memo No. 1330, 1991.
2. Larock, R., Comprehensive Organic Transformations, New York, NY, VCH Publishers, 1989, p. 432.
3. Mitwalli, A., Leeb, S., Tanaka, T., and Sinha, U, "Polymer Gel Actuators," Universities Power Engineering Conference, Galway, Ireland, 1994.
4. Ninomiya, K., Shioiri, T., and Yamada, S., "Phosphorus in Organic Synthesis - VII," Tetrahedron, Vol. 30, 1974, pp. 2151-2157.
5. Schmitz, F., and Yalamanchili, G., "Revised Structure of Bursatellin," J. Org. Chem., Vol. 52, 1987, pp. 2301-2303.
6. Umemoto, S., Okui, N., and Sakai, T., Polymer Gels, New York: Plenum Press, 1991, pp. 257-270.